Last ten years, my research interests is focusing on the Raman vibrational and the surface-enhanced Raman spectroscopy, as well as the surface plasmon resonance for the detection and identification of biomolecules (e.g. peptide, protein, DNA, viruses, antigens, antibodies, bacteria, fungi) for analytical and medical applications.
My biggest success in the area of SERS spectroscopy is fabrication and optimization of SERS–active nanostructures. A classical SERS substrates are based on three metals, Au, Ag, and Cu, which have localized surface plasmon resonances in the visible and near infrared spectral regions. An ideal SERS substrate should exhibits a uniform and high enhancement factor (EF), chemical stability, and the possibility to be produced cheaply and reproducibly. In recent years, many techniques have been used to prepare SERS substrates optimized in size, shape, and composition. Researchers have explored numerous promising substrates that could be used as efficient SERS-substrates. Although considerable development has been made towards improving and optimizing SERS substrates, the standard application of SERS in biomedical and analytical tests is still hindered by the lack of effective SERS substrates which would satisfy all the requirements mentioned above. However, during the last years my research group developed a several types of novel SERS substrates including electrochemically roughened electrodes[1], colloidal particle arrays[2], CuO[3], ZnO[4] and GaN films[5] but also polymer mat covered with the gold[6]. All these invented substrates showing a great potential to become excellent tools for biological or medical diagnostics:
(1) the novel copper-based SERS substrates were prepared using a new high pressure method, in which the decomposition of copper hydride is induced via pressure. This particular substrate show SERS enhancement factor of 107 and excellent reproducibility both across a single platform and among different platforms, and good performance in the detection of Staphylococcus aureus bacteria [3].
(2) the silver nanoparticle (AgNP) substrate was obtained in electrode- position onto tin-doped indium oxide using cyclic voltammetry. The substrates exhibit a SERS enhancement factor of around 107. The application of AuNP substrates as bio-sensors was demonstrated with the detection of the neurotransmitters, such as acetylcholine, dopamine, epinephrine and choline, the precursor for acetylcholine[7].
(3) an efficient and low-cost substrates based on Au coated zinc oxide layers with high enhancement factor of over 107. This SERS-active substrate exhibits good stability and reproducibility, and can be used for label–free SERS detection in both, biological and non-biological samples like neopterine [4].
(4) the novel Au-Ag coated GaN substrate were presented as SERS diagnostic platform for the detection of DNA mutations[8] – oligonucleotide sequences corresponding to the BCR-ABL (breakpoint cluster region-Abelson) gene responsible for development of chronic myelogenous leukemia, and was used as a model system to demonstrate the discrimination between the wild type and Met244Val mutations. Moreover, a sensitive, microfluidic SERS immunoassay using antigen-antibody interaction for detection of Hepatitis B virus antigen in blood[9] was also developed. The low detection limit for Hepatitis B virus antigen was estimated to be 0,01 IU/mL. This SERS immunoassay gives exact results over a broad linear range, reflecting clinically relevant HBsAg concentrations, and high biological specificity for the detection of Hepatitis B virus antigen.
(5) the SERS–active platform based onto silver-gold bimetallic surface prepared by electrochemical deposition of gold over an electrochemically roughened silver surface. The resultant substrate was very stable under atmospheric conditions and exhibited the strong Raman enhancement of 106 with the high reproducibility of the recorded SERS spectra of p-mercaptobenzoic acid and selected bacteria.
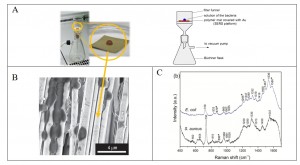
(6) the electrospun polymer mat as SERS platform especially for bacteria [6].
Presented above nanometallic structures for SERS analyzes were developed in our group, fully characterized, and described in ten international patents and four patent applications and at least fifteen papers published in high impact factor journals .
For example recently, we fill out three patent applications of SERS plasmonic nanostructures. The first patent innovated the SERS nanostructures build on ZnO thin layer deposited on silicon by Atomic Layer Deposition (ALD) method [10]. These SERS nanostructures were successfully applied for detection of immune markers like neopterine from blood samples. Next two patents described the SERS-active nanostructures dedicated especially for the bacteria detection from diluted solutions, e.g. urine, water or blood. They were based onto the Au or Au:Ag alloys coated polymer mats developed with force spinning process[11], and electrospinning method[12].
[1] [a] A. Sivanesan, E. Witkowska, W. Adamkiewicz, Ł. Dziewit, A. Kamińska*, J. Waluk, “Nanostructured Silver-Gold Bimetallic SERS Substrate for Selective Identification of Bacteria in Human Blood“, Analyst 139, 1037-43 (2014); 38; [b] A. Kamińska*, A. Kowalska, , P. Albrycht, and J. Waluk, „The ABO blood groups antigen-antibody interactions studied by SERS spectroscopy:towards the blood group typing” – Anal. Methods, 2016, DOI:10.1039/C5AY02658J
[2] A. Kaminska, R. J. Forster and T. E. Keyes, “The impact of adsorption of bovine pancreatic trypsin inhibitor on CTAB-protected gold nanoparticle arrays: a Raman spectroscopic comparison with solution denaturation”, Journal of Raman Spectroscopy, 41, 130-1334 (2009).
[3] A. Kowalska, A. Kaminska, W. Adamkiewicz, E.Witkowska and M.Tkacz, “Novel highly sensitive Cu-based SERS platforms for biosensing applications”, J. Raman Spectrosc., 46, 428–433 (2015).
[4] A. Kamińska*, A. A. Kowalska, D. Snigurenko, E. Guziewicz, J. Lewiński, J. Waluk, „ZnO oxide films for ultrasensitive, rapid, and label-free detection of neopterin by surface-enhanced Raman spectroscopy” Analyst 140, 5090-5098 (2015).
[5] A. Kamińska*, I. Dzięcielewski, J. L. Weyher, J. Waluk, S. Gawinkowski, V. Sashuk, M. Fiałkowski, M. Sawicka, T. Suski, S. Porowski and R. Hołyst “Highly reproducible, stable and multiply-regenerated Surface-Enhanced Raman Scattering substrate for biomedical applications”, Journal of Material Chemistry, 21, 8662 (2011)
[6] T. Szymborski, E. Witkowska, W. Adamkiewicz, J. Waluk and A. Kaminska* “Electrospun polymer mat as a SERS platform for immobilization and detection of bacteria from fluids “, Analyst, 139, 5061-5064 (2014).
[7] M. Siek, A. Kaminska*, A. Kelm, T. Rolinski, R. Holyst, M. Opallo and J. Niedziolka-Jönsson, “Electrodeposition for Preparation of Efficient SERS-Active Silver Nanoparticle Substrates for Neurotransmitter Detection “, Electrochimica Acta, 89, 284–291(2013).
[8] A. Kamińska*, A. Sivanesan, E. Witkowska, J. Gołąb, M. Winiarska, D. Nowis, I. Dzięcielewski, J. L. Weyher and J. Waluk, “ Detection of DNA Mutations Using Novel Surface-Enhanced Raman Spectroscopy (SERS) Diagnostic Platform”, J. Chem. Chem. Eng. 7, 972-978 (2013).
[9] A. Kamińska*, E. Witkowska, K. Winkler, I. Dzięcielewski, J. L. Weyher, and J. Waluk “Detection of Hepatitis B virus antigen from human blood: SERS immunoassay in a microfluidic system “, Biosensors and Bioelectronics 66,461–467 (2015).
[10] E. Guziewicz, D. Snigurenko, E. Witkowska, T. Szymborski and A. Kamińska-Michota 'A method for preparing a platform for testing of chemicals via surface-enhanced Raman spectroscopy (SERS) and platform obtained by this method' (number: P-406026)
[11] E. Witkowska, T. Szymborski, J. Waluk, and A. Kamińska 'The platform for testing chemicals and microorganisms via Surface Enhanced Raman Spectroscopy and method of preparation of the platform' (number: P-409210)
[12] T. Szymborski, E. Witkowska, W. Adamkiewicz, J. Waluk, and A. Kamińska 'The platform and its use for the detection and/or identification of microorganisms, especially bacteria, via the technique of surface-enhanced Raman spectroscopy (SERS) and a method for the deposition of these microorganisms on produced platforms' (number: P-406900)